A Paradigm Shift in Huntington’s Treatment
In a groundbreaking development that’s being called “world-changing” by leading researchers, a new experimental gene therapy slashes Huntington’s progression by 75%. Its called: AMT-130 has demonstrated an unprecedented reduction in Huntington’s disease progression over three years in patients who received a high dose.
This marks the first time any treatment has shown the ability to actually slow the progression of this devastating genetic disorder, rather than just manage its symptoms.
Professor Sarah Tabrizi from University College London (UCL), who has been studying potential therapies for Huntington’s for two decades, described the results as “huge,” stating she has “never seen anything that shows that [benefit].”
Professor Ed Wild, principal investigator at the UCL Huntington’s Disease Centre, went even further, declaring: “This result changes everything. On the basis of these results it seems likely AMT-130 will be the first licensed treatment to slow Huntington’s disease, which is truly world-changing stuff.”
Understanding the Breakthrough
The AMT-130 gene therapy, developed by Netherlands and USA-based company uniQure, achieved a statistically significant 75% slowing of disease progression as measured by the composite Unified Huntington’s Disease Rating Scale (cUHDRS), which incorporates motor, cognitive and functional measures.
One remarkable success story involves a patient who had to medically retire due to the disease but has been able to return to work after treatment.
The treatment works by delivering a therapeutic gene directly to affected brain regions – specifically the putamen and caudate nucleus – using a one-time surgical procedure.
Unlike traditional medications, AMT-130 uses RNA interference to silence the huntingtin gene, reducing production of the toxic protein that causes the disease.
Get Copay Assistance
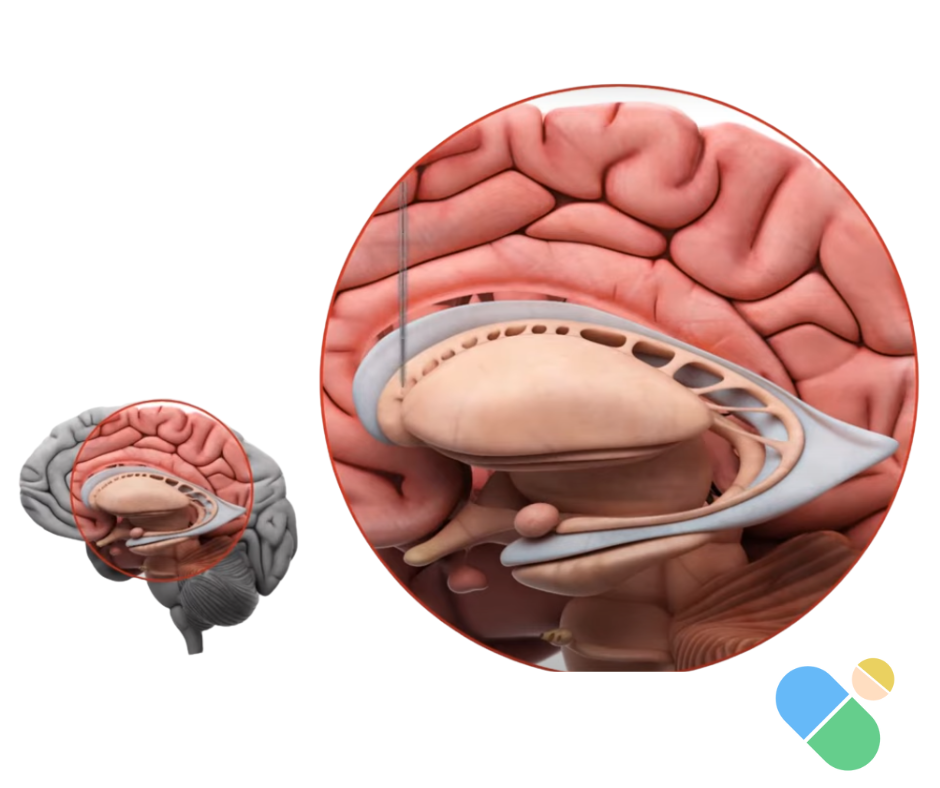
Cross-sectional view of the human brain highlighting the striatum – the primary target for AMT-130 gene therapy. The treatment is surgically delivered to the caudate nucleus and putamen, regions most affected by Huntington’s disease, where it silences the toxic huntingtin gene.
What This Means for People Living with Huntington’s
Amy Gray, president and CEO of the Huntington’s Disease Society of America, called this “a truly transformative development” for the approximately 42,000 Americans living with the condition, noting that “for decades, there have been no therapies to slow disease progression—only treatments to manage symptoms.”
If approved, AMT-130 would become the first treatment to actually slow down Huntington’s disease rather than just treat its symptoms.
The US FDA has already granted both Breakthrough Therapy designation and Regenerative Medicine Advanced Therapy designation, which could expedite the approval process.
UniQure plans to submit their Biologics License Application to the FDA in the first quarter of 2026.
The Future of Medicine: Gene Therapy’s Coming of Age
This breakthrough represents a watershed moment not just for Huntington’s disease, but for the entire field of gene therapy and neurodegenerative medicine. The study demonstrates that gene therapy is becoming a powerful way to treat challenging diseases that don’t respond to traditional treatments.
The implications extend far beyond Huntington’s:
- Proof of concept for treating other genetic neurodegenerative diseases
- Validation of gene silencing as a therapeutic approach
- Hope for conditions previously considered untreatable
- Acceleration of research into similar therapies for other disorders
Additionally, another promising trial called ALN-HTT02 has just begun, targeting a specific region of the HTT gene called exon 1, which researchers believe is a key driver of the disease’s toxicity.
This represents a new wave of precision treatments on the horizon.
Current Symptom Management: The Role of Tetrabenazine

Managing Huntington’s Chorea: The Critical Role of Tetrabenazine in Current Treatment
While we await the availability of these groundbreaking gene therapies, current treatment options remain crucial for managing the debilitating symptoms of Huntington’s disease. This is where medications like tetrabenazine play a vital role in improving patients’ quality of life today.
How Tetrabenazine Provides Relief
Tetrabenazine (TBZ) works as a selective inhibitor of vesicular monoamine transporter 2 (VMAT2), depleting presynaptic dopamine, norepinephrine, and serotonin storage to reduce the involuntary choreic movements that characterize Huntington’s disease.
It was the first FDA-approved treatment specifically for Huntington’s disease chorea in the United States (approved in 2008).
In the pivotal TETRA-HD clinical trial, tetrabenazine treatment resulted in a reduction of 5.0 units in chorea severity on the Unified Huntington’s Disease Rating Scale (UHDRS) compared with only 1.5 units on placebo – a statistically significant improvement.
The duration of effect from a single dose typically lasts approximately 5 hours, with chorea scores decreasing on average by 42.4%.
Impact on Quality of Life
Long-term studies have shown that tetrabenazine effectively suppresses HD-related chorea for up to 80 weeks and beyond, with participants experiencing overall improvement as demonstrated by Clinical Global Impression scores.
Importantly, managing chorea can help reduce embarrassment, frustration, and physical harm from falls or injuries, significantly impacting patients’ quality of life.
Julia Kravtsova, Lead Pharmacist at QuickRx Specialty Pharmacy, emphasizes the importance of symptom management: “While we eagerly await the availability of disease-modifying therapies like AMT-130, medications like tetrabenazine remain absolutely essential for our patients. Every day we see how controlling chorea allows people to maintain their dignity, continue working longer, and preserve precious time with their families.”
Though life expectancy after diagnosis typically ranges from 15-20 years, with juvenile onset cases having shorter prognoses of about 10 years, effective symptom management with medications like tetrabenazine can significantly improve the quality of those years.
Studies confirm that tetrabenazine does not accelerate disease progression and allows patients to maintain cognitive and functional abilities at rates consistent with the natural history of HD.
QuickRx’s Commitment to Access
At QuickRx Specialty Pharmacy, we understand the challenges families face when dealing with Huntington’s disease. That’s why we offer comprehensive support for tetrabenazine access, including:
- Copay Assistance Programs to reduce out-of-pocket costs
- 24/7 Support for questions and concerns
- Nationwide Delivery to ensure continuous access to medication
- Insurance Navigation to maximize coverage benefits
Kravtsova adds: “We’ve seen firsthand how tetrabenazine can be life-changing for patients struggling with chorea. Our role is to ensure that financial barriers never prevent someone from accessing this vital medication. The bridge between today’s symptom management and tomorrow’s gene therapies is critical, and we’re committed to helping patients cross it.”
Need Tetrabenazine Copay Assistance?
Timeline for Hope: When AMT-130 Will Reach Patients

Racing Toward 2026: AMT-130 gene therapy advances through final stages before FDA approval for Huntington’s disease
US Market Timeline
UniQure plans to submit their Biologics License Application (BLA) to the FDA in the first quarter of 2026.
If approved, the treatment could potentially launch later in 2026.
The FDA’s grant of both Breakthrough Therapy and Regenerative Medicine Advanced Therapy designations should help expedite the review process.
European/UK Market Timeline
Applications for UK and European approval will follow after the US FDA submission, suggesting availability in these markets could come in late 2026 or 2027.
The Road Ahead: Key Milestones
2025-2026:
- Q1 2026: FDA application submission
- Mid-2026: Anticipated FDA review completion
- Late 2026: Potential US market launch
2027 and Beyond:
- European and UK approvals expected
- Expansion to other global markets
- Treatment center certification and surgeon training
- Insurance coverage determinations
While the standard FDA approval process typically takes several years of additional testing and review, the breakthrough designations and compelling efficacy data may accelerate availability. However, patients should realistically prepare for a 1-3 year timeline before treatment becomes widely accessible, considering manufacturing scale-up, specialized surgical training requirements, and insurance negotiations.
Looking Ahead: A New Era of Hope
The convergence of breakthrough gene therapies and improved symptom management represents a turning point in the fight against Huntington’s disease. While AMT-130 and other gene therapies promise to fundamentally change the disease trajectory, current treatments like tetrabenazine continue to provide essential relief for patients today.
As Professor Tabrizi notes, these results are “the most convincing evidence in the field to date” and “underscore the disease-modifying effect in Huntington’s disease, where an urgent need persists.”
The extraordinary bravery of trial participants who underwent major neurosurgery to test the first gene therapy for Huntington’s disease represents “an extraordinary act of bravery for the benefit of humanity.”
The medical community stands at the threshold of a new era – one where genetic diseases once considered death sentences may become manageable conditions, and where the gap between diagnosis and disability can be dramatically extended. For the thousands of families affected by Huntington’s disease, this breakthrough offers something that has been in short supply: genuine hope for a different future.
Resources and Further Reading
- UCL Gene Therapy Study Results
- UniQure AMT-130 Program Information
- FDA Tetrabenazine Information
- QuickRx Tetrabenazine Support Program
- Huntington’s Disease Society of America
For more information about tetrabenazine access and support programs, contact QuickRx Specialty Pharmacy at (917) 830-2525 or visit quickrxspecialty.pharmacy/tetrabenazine.