⚠️ Medical Disclaimer: This article is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always consult your oncologist or healthcare provider before making any changes to your treatment plan. The information below is sourced from peer-reviewed pharmaceutical references and reviewed by a licensed pharmacist.
Key Takeaways
- Hypertension is the most common Lenvima side effect, affecting up to 73% of patients — regular blood pressure monitoring is essential
- Fatigue, diarrhea, and decreased appetite are reported in more than 30% of patients across all cancer types
- Most side effects are manageable with dose adjustments, supportive medications, and close monitoring by your oncology team
- Serious side effects like cardiac toxicity, hemorrhage, and liver injury require immediate medical attention
- QuickRx Specialty Pharmacy provides free Lenvima copay assistance to help make treatment affordable — call (917) 830-2525
Quick Navigation
Starting a new cancer medication can feel overwhelming — especially when you read the list of potential side effects. If your oncologist has prescribed Lenvima (lenvatinib), understanding what to expect can help you feel more prepared and in control of your treatment journey.
Lenvima is a powerful targeted therapy used to treat several types of cancer, and like all cancer medications, it comes with potential side effects. The good news? Most Lenvima side effects are manageable with the right support from your oncology team.
“One of the most important things patients can do when starting Lenvima is to understand the side effect profile ahead of time. When patients know what to watch for, they can report symptoms early — and early intervention often means the difference between managing a side effect and having to interrupt treatment.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
What Is Lenvima (Lenvatinib)?
Lenvima (lenvatinib) is an FDA-approved oral cancer medication classified as a multitargeted tyrosine kinase inhibitor. First approved on February 13, 2015, and manufactured by Eisai Inc., Lenvima works by blocking multiple proteins involved in tumor growth and the formation of new blood vessels that feed cancer cells.
Lenvima is currently FDA-approved to treat four types of cancer:
- Differentiated thyroid cancer — for radioactive iodine-refractory disease (24 mg daily)
- Hepatocellular carcinoma (liver cancer) — as first-line monotherapy (8–12 mg daily based on weight)
- Renal cell carcinoma (kidney cancer) — in combination with pembrolizumab or everolimus (18–20 mg daily)
- Endometrial carcinoma — in combination with pembrolizumab for pMMR/not MSI-H tumors (20 mg daily)
Because Lenvima targets multiple pathways simultaneously, its side effect profile reflects its broad mechanism of action. The type and severity of side effects can vary depending on which cancer is being treated, the dose prescribed, and whether Lenvima is used alone or in combination with other medications.
Common Lenvima Side Effects
Clinical trials have identified several side effects that occur frequently in patients taking Lenvima. The percentages below reflect data across multiple cancer types and may vary by indication.
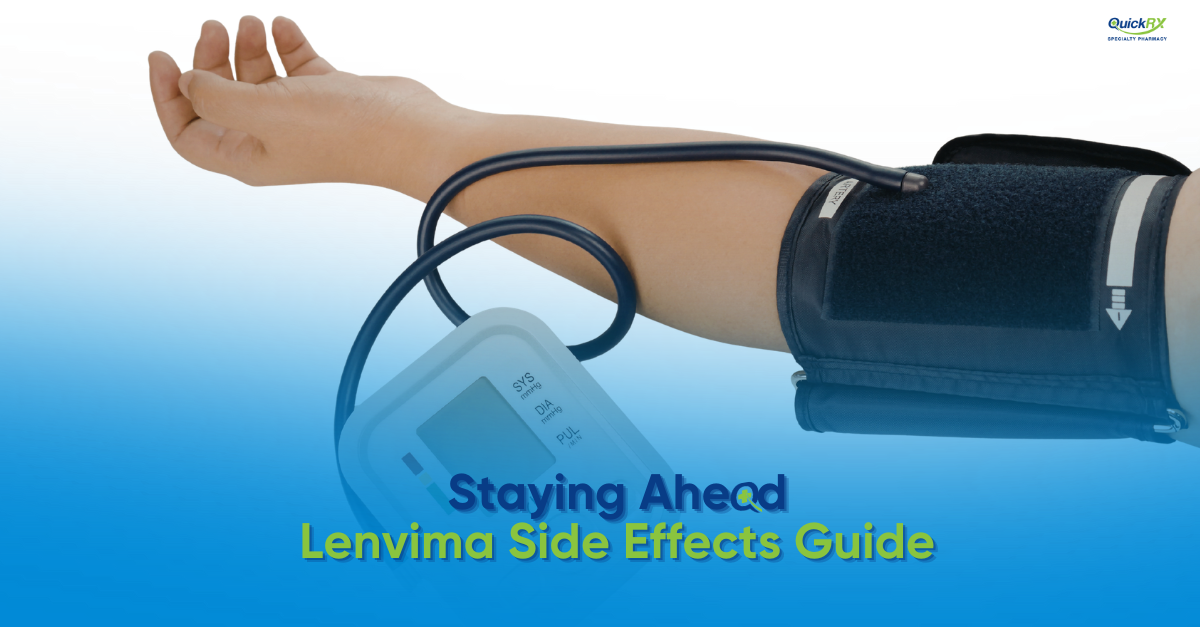
High Blood Pressure (Hypertension) — 45% to 73%
Hypertension is the most commonly reported Lenvima side effect and occurs across all approved indications. Blood pressure elevation can happen within the first week of treatment and requires regular monitoring. Your oncologist will typically check blood pressure after 1 week, every 2 weeks for the first 2 months, and then at least monthly thereafter. Blood pressure medications may be started or adjusted during Lenvima treatment.
Fatigue — 44% to 67%
Fatigue is one of the most impactful side effects for patients on Lenvima. It can range from mild tiredness to significant exhaustion that affects daily activities. Fatigue may be related to the medication itself, thyroid dysfunction (hypothyroidism is also common with Lenvima), or the cancer being treated.
Diarrhea — 39% to 67%
Diarrhea is very common with Lenvima treatment and can range from mild to severe. Grades 3 and 4 diarrhea (severe) have been reported in 4% to 9% of patients. Staying hydrated and having anti-diarrheal medications available is important. Notify your oncology team if diarrhea becomes severe or persistent.
Decreased Appetite and Weight Loss — 34% to 54% and 31% to 51%
Many patients experience reduced appetite while taking Lenvima, which can lead to significant weight loss over time. Nutritional support and dietary counseling may help maintain adequate caloric intake during treatment.
Nausea and Vomiting — 20% to 47% and 16% to 36%
Lenvima is associated with moderate to high emetic potential. Your oncologist may prescribe anti-nausea medications (antiemetics) to take alongside your Lenvima treatment. Taking Lenvima at a consistent time each day may also help manage nausea.
Hand-Foot Syndrome (Palmar-Plantar Erythrodysesthesia) — 27% to 32%
Hand-foot syndrome causes redness, swelling, pain, and peeling on the palms of the hands and soles of the feet. This side effect can impact daily activities and quality of life. Moisturizing creams, avoiding hot water, and wearing comfortable shoes can help manage symptoms.
Mouth Sores (Stomatitis) — 11% to 41%
Inflammation and sores in the mouth and throat are common with Lenvima. Good oral hygiene, gentle mouthwashes, and avoiding spicy or acidic foods can help. Your doctor may prescribe medicated mouth rinses for more severe cases.
Proteinuria (Protein in Urine) — 26% to 34%
Lenvima can affect kidney function, causing protein to leak into the urine. Your oncology team will monitor this with regular urine tests. If proteinuria becomes significant (2+ on dipstick), a 24-hour urine collection may be needed.
Hypothyroidism (Underactive Thyroid) — Approximately 21%
Lenvima can affect thyroid function, leading to an underactive thyroid. Symptoms include increased fatigue, weight gain, cold intolerance, and constipation. Thyroid function (TSH) is monitored at baseline and monthly during treatment, and thyroid replacement medication may be prescribed if needed.
“Many of the common Lenvima side effects — like hypertension, diarrhea, and fatigue — are very manageable when patients and their care teams are proactive. The key is consistent monitoring and open communication. Don’t wait until a side effect becomes severe to report it.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Serious Lenvima Side Effects to Watch For
While less common, Lenvima can cause serious side effects that require prompt medical attention. Being aware of these risks allows you to seek help quickly if they occur.
Cardiac Toxicity
Lenvima has been associated with heart problems including cardiomyopathy, heart failure, ventricular dysfunction, and QT interval prolongation. Patients with pre-existing heart conditions, those taking other QT-prolonging medications, or those with electrolyte imbalances may be at higher risk. ECG monitoring may be required in select patients.
Hemorrhage (Bleeding)
Serious bleeding events, including fatal hemorrhage and tumor hemorrhage, have been reported with Lenvima. Bleeding can occur at any site. Contact your healthcare provider immediately if you experience unusual bleeding, blood in your stool or urine, or coughing up blood.
Hepatotoxicity (Liver Injury)
Serious liver problems, including hepatic failure, have been reported. Liver function tests are checked at baseline, every 2 weeks for the first 2 months, and then at least monthly. Patients with hepatocellular carcinoma should be monitored especially closely for signs of hepatic encephalopathy.
Arterial Thromboembolic Events
Blood clots in the arteries, including heart attacks and strokes, have been reported in 2% to 5% of patients taking Lenvima. Seek emergency medical care if you experience sudden chest pain, shortness of breath, weakness on one side of the body, or difficulty speaking.
GI Perforation and Fistula Formation
Holes or abnormal connections in the digestive tract are rare but serious complications. Severe abdominal pain, fever, or significant changes in bowel habits should be reported to your doctor immediately.
Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
This rare neurological condition can cause headache, seizures, confusion, and visual disturbances. If you experience any of these symptoms, seek immediate medical attention.
Wound Healing Impairment
Lenvima can impair the body’s ability to heal wounds. For this reason, treatment should be withheld for at least 1 week before elective surgery and should not be resumed for at least 2 weeks after major surgery until adequate wound healing has occurred.
🚨 When to Call Your Doctor Immediately
Contact your oncology team or seek emergency medical care right away if you experience any of the following while taking Lenvima:
- Unusual bleeding or bruising — blood in stool/urine, nosebleeds that won’t stop, coughing up blood
- Chest pain or shortness of breath — could indicate cardiac problems or blood clots
- Severe headache, confusion, or seizures — could indicate RPLS or severely elevated blood pressure
- Sudden vision changes — blurred vision, loss of vision
- Severe abdominal pain — could indicate GI perforation
- Signs of liver problems — yellowing of skin/eyes (jaundice), dark urine, severe nausea
- Signs of stroke — sudden weakness on one side, difficulty speaking, facial drooping
- Severe or persistent diarrhea — more than 6 episodes per day or signs of dehydration
- Wounds that won’t heal — especially after surgery or dental procedures
Do not wait to see if symptoms improve on their own. Early intervention is critical with Lenvima-related complications.
Managing Lenvima Side Effects: Practical Tips
The good news is that most Lenvima side effects can be managed effectively with proactive care, regular monitoring, and open communication with your oncology team. Here are some practical strategies:
Stay on Top of Blood Pressure Monitoring
Since hypertension is the most common Lenvima side effect, consider investing in a home blood pressure monitor. Check your blood pressure at the same time each day and keep a log to share with your doctor. If your blood pressure is consistently elevated, your doctor may adjust your medications. The American Heart Association provides helpful guidance on blood pressure readings.
Manage Diarrhea Proactively
Keep anti-diarrheal medications on hand (ask your oncologist which ones are appropriate). Stay well-hydrated with clear fluids, broths, and electrolyte drinks. Eat small, frequent meals and avoid high-fiber, greasy, or spicy foods during flare-ups. Track the frequency and severity of episodes to report to your care team.
Combat Fatigue Strategically
Plan your most important activities during your highest-energy times of day. Short walks and light exercise can actually help combat cancer-related fatigue. Ensure your thyroid function is being checked monthly, as hypothyroidism can worsen fatigue. The National Cancer Institute’s fatigue guide offers additional coping strategies.
Protect Your Hands and Feet
For hand-foot syndrome, apply thick moisturizing creams (like urea-based creams) to hands and feet regularly. Avoid hot water, tight shoes, and repetitive friction. Wear cotton socks and gloves at night after moisturizing. Report early symptoms to your doctor — dose adjustments may help prevent worsening.
Maintain Good Oral Hygiene
Brush with a soft-bristled toothbrush and use alcohol-free mouthwash. Avoid spicy, acidic, or crunchy foods that can irritate mouth sores. Stay hydrated. Your doctor may prescribe medicated mouth rinses or oral analgesics if stomatitis becomes painful. Remember: a preventive dental exam before starting Lenvima is recommended.
Keep All Monitoring Appointments
Regular lab work is essential during Lenvima treatment. Blood pressure, liver function, kidney function, thyroid levels, electrolytes, and urine protein all need to be monitored on schedule. Skipping appointments can mean missed warning signs. Your oncology team uses these results to catch problems early and keep your treatment on track.
Dose Adjustments Are Normal
Your oncologist may reduce your Lenvima dose if side effects become too severe. This is a normal part of cancer treatment management — not a sign of failure. Lenvima is available in multiple dose pack configurations (4 mg through 24 mg) specifically to allow for flexible dose adjustments.
💚 Affording Lenvima Treatment
Managing side effects is only part of the challenge — the cost of Lenvima can also be a significant burden. As a specialty medication with no generic version currently available, Lenvima can be very expensive without financial assistance.
QuickRx Specialty Pharmacy provides completely free Lenvima copay assistance to help cancer patients access affordable treatment. Our services include:
- Manufacturer copay card enrollment for commercially insured patients
- Patient assistance program coordination for uninsured or underinsured patients
- Foundation grant applications for cancer patients
- Insurance navigation and prior authorization support
- Temperature-controlled home delivery nationwide (licensed in all 50 states)
Call QuickRx today: (917) 830-2525 | Toll-free: (800) 496-6111
“No patient should have to choose between managing their cancer and affording their medication. At QuickRx, we work with every patient individually to find the best financial assistance option — whether that’s a manufacturer copay card, a patient assistance program, or a foundation grant. The process is free, and our team handles all the paperwork.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Frequently Asked Questions About Lenvima Side Effects
1. What is the most common side effect of Lenvima?
Hypertension (high blood pressure) is the most commonly reported Lenvima side effect, affecting 45% to 73% of patients depending on the cancer type being treated. Regular blood pressure monitoring — at home and during clinic visits — is essential throughout treatment.
2. Does Lenvima cause hair loss?
Hair loss (alopecia) has been reported in some patients taking Lenvima, though it is not among the most common side effects. The rate varies by indication. Talk to your oncology team if you experience changes in hair growth during treatment.
3. How long do Lenvima side effects last?
Some Lenvima side effects like nausea and fatigue may improve as your body adjusts to the medication over the first few weeks. Other side effects like hypertension may persist throughout treatment and require ongoing management. If Lenvima is discontinued, most side effects resolve within days to weeks.
4. Can Lenvima side effects be managed without stopping treatment?
Yes, most Lenvima side effects can be managed through supportive medications, dose adjustments, and lifestyle modifications without interrupting treatment entirely. Your oncologist has multiple tools available, including dose reductions across several available dose pack configurations.
5. Does Lenvima affect thyroid function?
Yes. Hypothyroidism (underactive thyroid) occurs in approximately 21% of patients taking Lenvima. Your TSH levels will be monitored at baseline and monthly. If hypothyroidism develops, thyroid replacement hormone medication may be prescribed.
6. Are Lenvima side effects different depending on cancer type?
Yes, the frequency and severity of certain side effects can vary depending on which cancer is being treated and whether Lenvima is used as monotherapy or in combination with other medications like pembrolizumab or everolimus. Your oncologist can discuss the specific side effect profile relevant to your treatment plan.
7. Can I take Lenvima with blood pressure medication?
Yes, and many patients do. Since hypertension is so common with Lenvima, your doctor may start or adjust blood pressure medications during treatment. Always inform your oncology team about all medications you take, as some may interact with Lenvima.
8. What should I do about hand-foot syndrome from Lenvima?
For hand-foot syndrome, use thick moisturizing creams regularly, avoid hot water and tight shoes, and wear cotton socks and gloves at night. Report symptoms early to your doctor — dose adjustments can often help before symptoms become severe.
9. Does Lenvima interact with food?
Lenvima can be taken with or without food. However, high-fat meals (approximately 900 calories with about 55% fat, 15% protein, and 30% carbohydrates) may delay the rate of absorption and delay the time to peak concentration from 2 hours to 4 hours. Consistent dietary habits are recommended.
10. How can I afford Lenvima if the side effects mean I need ongoing treatment adjustments?
QuickRx Specialty Pharmacy provides free Lenvima copay assistance regardless of dose changes or treatment adjustments. Whether you’re on 24 mg or 4 mg, our team helps you access manufacturer copay cards, patient assistance programs, and foundation grants. Call (917) 830-2525 to get started.
Author: Paola Larrabure, Pharma Content Manager at QuickRx Specialty Pharmacy
Medically Reviewed by: Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Last Updated: February 2026
References
- Lenvatinib. Lexi-Drugs. Lexicomp. UpToDate, Inc. Accessed February 13, 2026.
- National Cancer Institute. Lenvatinib Mesylate — NCI Drug Information.
- U.S. Food and Drug Administration. FDA Approved Drug Products: Lenvima (lenvatinib).
- National Cancer Institute. Side Effects of Cancer Treatment.
- National Cancer Institute. Fatigue and Cancer Treatment.
- American Heart Association. Understanding Blood Pressure Readings.
- Mayo Clinic. Long QT Syndrome.
- American Cancer Society. Cancer.org — Patient Education Resources.
Comprehensive Medical Disclaimer: This article is published by QuickRx Specialty Pharmacy for educational and informational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or medication. Never disregard professional medical advice or delay in seeking it because of something you have read in this article. QuickRx Specialty Pharmacy does not recommend or endorse any specific tests, physicians, products, procedures, opinions, or other information that may be mentioned in this article. Reliance on any information provided in this article is solely at your own risk.
HIPAA Notice: QuickRx Specialty Pharmacy is committed to protecting your privacy. All patient information is handled in accordance with HIPAA regulations. View our Privacy Policy.
Lenvima® is a registered trademark of Eisai Inc. QuickRx Specialty Pharmacy is not affiliated with Eisai Inc.
================================================================================
FAQ SCHEMA MARKUP (JSON-LD) – Add via WPCode or Custom HTML block
================================================================================