⚠️ Medical Disclaimer: This article is for educational purposes only and is not a substitute for professional medical advice, diagnosis, or treatment. Always consult your oncologist or endocrinologist before making any changes to your treatment plan. The information below is sourced from peer-reviewed pharmaceutical references and reviewed by a licensed pharmacist.
Key Takeaways
- Lenvima (lenvatinib) is FDA-approved for differentiated thyroid cancer that no longer responds to radioactive iodine treatment
- The standard dose for thyroid cancer is 24 mg once daily — the highest of all Lenvima indications
- Lenvima works by blocking multiple proteins that help tumors grow and form new blood vessels
- Common side effects include hypertension, fatigue, diarrhea, and decreased appetite — most are manageable with proper care
- There is no generic lenvatinib available — copay assistance is critical for managing treatment costs
- QuickRx provides free Lenvima copay assistance — call (917) 830-2525
Quick Navigation
A thyroid cancer diagnosis can be life-changing, and when standard treatments like surgery and radioactive iodine are no longer effective, learning about your next treatment options becomes especially important. If your endocrinologist or oncologist has mentioned Lenvima (lenvatinib) as a treatment option, this guide will help you understand what it is, how it works, what to expect, and how to access financial assistance.
“Being diagnosed with radioactive iodine-refractory thyroid cancer can feel especially frustrating because patients have already been through so much. Lenvima offers a meaningful treatment option for these patients, and understanding how it works — and how to manage it — can make a real difference in their experience and outcomes.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Understanding Differentiated Thyroid Cancer
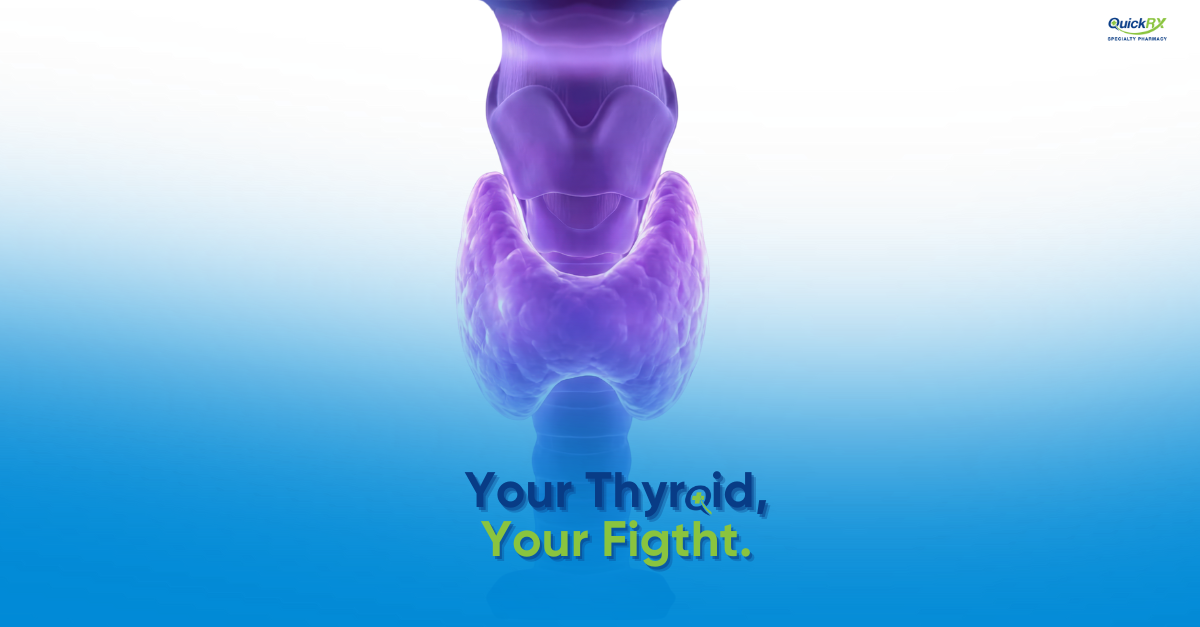
Thyroid cancer begins in the thyroid gland, a butterfly-shaped gland at the base of the neck that produces hormones regulating metabolism, heart rate, and body temperature. Differentiated thyroid cancer (DTC) is the most common type, accounting for the vast majority of thyroid cancer cases.
Types of Differentiated Thyroid Cancer
Differentiated thyroid cancer includes two main subtypes:
- Papillary thyroid cancer — the most common type, making up approximately 80% of all thyroid cancers
- Follicular thyroid cancer — the second most common type, accounting for approximately 10-15% of cases
These cancers are called “differentiated” because the cancer cells still resemble and behave somewhat like normal thyroid cells, which is generally a favorable characteristic. Most patients with differentiated thyroid cancer have an excellent prognosis with standard treatment.
Standard Treatment Pathway
The typical treatment journey for differentiated thyroid cancer follows a general progression:
- Surgery (thyroidectomy) — removal of part or all of the thyroid gland
- Radioactive iodine (RAI) therapy — uses radioactive iodine to destroy remaining thyroid cancer cells
- Thyroid hormone replacement — lifelong medication to replace hormones and suppress TSH
- Monitoring — regular blood tests and imaging to detect recurrence
When Standard Treatment Is No Longer Enough
While most patients respond well to surgery and radioactive iodine, a subset of patients develop cancer that becomes radioactive iodine-refractory (RAI-refractory). This means the cancer cells no longer absorb radioactive iodine effectively, making RAI therapy ineffective. When this happens — and the cancer continues to grow or spread — systemic therapies like Lenvima become an important treatment option.
According to the National Cancer Institute, RAI-refractory differentiated thyroid cancer represents a significant clinical challenge, and targeted therapies have transformed the treatment landscape for these patients.
What Is Lenvima (Lenvatinib)?
Lenvima (lenvatinib) is an oral cancer medication classified as a multitargeted tyrosine kinase inhibitor (TKI). It was approved by the FDA on February 13, 2015, for the treatment of radioactive iodine-refractory differentiated thyroid cancer — making it one of the first targeted therapies specifically approved for this indication.
Lenvima is manufactured by Eisai Inc. and is available only through specialty pharmacies. There is currently no generic version of lenvatinib available in the United States.
Unlike chemotherapy, which affects rapidly dividing cells throughout the body, Lenvima is a targeted therapy that specifically blocks certain proteins involved in cancer growth. This targeted approach is why Lenvima’s side effect profile, while significant, is different from traditional chemotherapy.
Who Qualifies for Lenvima Treatment?
Lenvima is specifically indicated for adult patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer. In practical terms, this means:
- Locally recurrent or metastatic: The cancer has come back in the thyroid area or has spread to other parts of the body (such as the lungs, bones, or lymph nodes)
- Progressive: The cancer is actively growing despite previous treatment
- Radioactive iodine-refractory: The cancer no longer responds to radioactive iodine therapy
Your oncologist or endocrinologist will determine whether Lenvima is appropriate for your specific situation based on your cancer’s characteristics, your overall health, and your treatment history.
How Lenvima Works Against Thyroid Cancer
Lenvima works by simultaneously blocking multiple receptor tyrosine kinases — proteins on the surface of cells that play a role in tumor growth and the formation of new blood vessels (angiogenesis).
The Targets Lenvima Blocks:
- VEGF receptors (VEGFR1, VEGFR2, VEGFR3): These receptors drive the formation of new blood vessels that supply tumors with nutrients and oxygen. By blocking them, Lenvima starves the tumor of its blood supply.
- FGF receptors (FGFR1–FGFR4): These receptors promote cell growth and survival. Blocking them directly inhibits tumor cell proliferation.
- PDGFR-alpha: Involved in the development of blood vessel support cells.
- RET: A protein commonly associated with thyroid cancer development. Mutations in RET are found in some thyroid cancers, making Lenvima’s inhibition of RET particularly relevant for thyroid cancer patients.
- KIT: Another growth factor receptor involved in cell signaling.
By targeting multiple pathways at once, Lenvima attacks cancer through two main mechanisms: cutting off the tumor’s blood supply (anti-angiogenic effect) and directly slowing cancer cell growth (antiproliferative effect). This dual approach is what makes Lenvima effective even in cancers that have become resistant to other treatments.
Lenvima Dosing for Thyroid Cancer
For differentiated thyroid cancer, the standard Lenvima dose is 24 mg taken orally once daily. This is the highest dose used across all of Lenvima’s approved indications.
Important Dosing Details:
- Take Lenvima at the same time each day, with or without food
- Swallow capsules whole — do not crush or chew
- If you cannot swallow capsules, Lenvima can be dissolved in a small amount of liquid to create a suspension
- The 24 mg daily dose is typically supplied as a combination of 10 mg and 4 mg capsules
- If you miss a dose and it’s within 12 hours of your scheduled time, take it. If more than 12 hours have passed, skip it and take the next dose on schedule
Dose Adjustments:
Your oncologist may reduce your Lenvima dose if you experience significant side effects. Dose reductions typically follow a stepwise approach (from 24 mg down to 20 mg, 14 mg, 10 mg, or lower as needed). Lenvima is available in multiple capsule strengths specifically to allow for these adjustments.
Patients with severe kidney impairment (CrCl less than 30 mL/min) may start at a reduced dose of 14 mg daily. Patients with severe liver impairment (Child-Pugh class C) may also require a reduced starting dose of 14 mg daily.
Side Effects to Expect with Lenvima for Thyroid Cancer
Because the thyroid cancer dose (24 mg) is the highest of all Lenvima indications, patients may experience side effects more frequently or at greater intensity. The most common side effects reported in thyroid cancer clinical trials include:
- Hypertension (high blood pressure): Very common — requires regular monitoring and may need blood pressure medication
- Fatigue: Very common — can significantly affect daily life
- Diarrhea: Very common — keep anti-diarrheal medications available
- Decreased appetite and weight loss: Very common — nutritional counseling may help
- Nausea and vomiting: Common — anti-nausea medications may be prescribed
- Mouth sores (stomatitis): Common — maintain good oral hygiene
- Hand-foot syndrome: Common — use moisturizing creams on hands and feet
- Proteinuria: Common — monitored through regular urine tests
For a more detailed breakdown of Lenvima side effects and management strategies, read our comprehensive guide: Lenvima Side Effects: What Every Cancer Patient Should Know.
“For thyroid cancer patients on the 24 mg dose, being proactive about side effect management is essential. I always encourage patients to have their blood pressure cuff at home, keep a symptom diary, and call us if anything feels off. Early intervention is the best way to stay on treatment and get the best outcomes.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Monitoring During Lenvima Treatment
Lenvima requires close and regular monitoring throughout treatment. Your oncology team will typically schedule the following:
Blood Pressure Monitoring
Checked after 1 week of treatment, then every 2 weeks for the first 2 months, then at least monthly. Home monitoring is strongly recommended.
Liver Function Tests (LFTs)
Checked at baseline, every 2 weeks for the first 2 months, then at least monthly. These tests help detect liver problems early.
Thyroid Function (TSH)
Monitored at baseline and monthly. This is especially important for thyroid cancer patients who are already on thyroid hormone replacement therapy — Lenvima can further affect thyroid function and may require dose adjustments of your thyroid medication.
Kidney Function and Proteinuria
Kidney function is checked at baseline and periodically. Urine protein is monitored with a dipstick test; if protein levels are elevated (2+), a 24-hour urine collection may be ordered.
Electrolytes and Calcium
Checked at baseline and periodically, with calcium monitored at least monthly. Thyroid cancer patients who have had a thyroidectomy may already have calcium management needs that require close attention.
Cardiac Monitoring
ECG monitoring may be required for patients with pre-existing heart conditions, those on other QT-prolonging medications, or those with electrolyte abnormalities.
Dental Exams
A dental exam is recommended before starting Lenvima, with preventive dentistry and good oral hygiene maintained throughout treatment. Osteonecrosis of the jaw is a potential risk.
🚨 When to Seek Immediate Medical Attention
Contact your doctor or go to the emergency room right away if you experience:
- Unusual or severe bleeding
- Chest pain, shortness of breath, or racing heartbeat
- Severe headache, confusion, seizures, or vision changes
- Severe abdominal pain
- Yellowing of skin or eyes (jaundice)
- Signs of stroke — sudden weakness, difficulty speaking, facial drooping
- High fever or signs of infection
💚 Affording Lenvima for Thyroid Cancer
Lenvima is a specialty medication with no generic version currently available, which means treatment costs can be a significant barrier for many thyroid cancer patients. The good news is that financial assistance is available.
QuickRx Specialty Pharmacy provides completely free Lenvima copay assistance to help make treatment accessible and affordable:
- Manufacturer copay card enrollment for commercially insured patients
- Patient assistance programs for uninsured or underinsured patients
- Foundation grant applications for thyroid cancer patients
- Insurance navigation, prior authorization, and appeals support
- Temperature-controlled home delivery — nationwide (licensed in all 50 states)
We understand the unique needs of thyroid cancer patients and are here to support you throughout your treatment journey.
Call QuickRx today: (917) 830-2525 | Toll-free: (800) 496-6111
“Thyroid cancer patients dealing with radioactive iodine-refractory disease have usually been through a long treatment journey already. The last thing they need is to worry about how to pay for the next phase of treatment. That’s where we come in — our team handles all the financial assistance paperwork so patients can focus on their health.”
— Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Frequently Asked Questions About Lenvima for Thyroid Cancer
1. Is Lenvima a chemotherapy drug?
No. Lenvima is a targeted therapy, not traditional chemotherapy. While chemotherapy works by killing rapidly dividing cells throughout the body, Lenvima specifically blocks certain proteins (tyrosine kinases) involved in tumor growth and blood vessel formation. This targeted approach means Lenvima’s side effect profile is different from chemotherapy.
2. How long do patients typically take Lenvima for thyroid cancer?
Lenvima is generally taken continuously until the cancer progresses or side effects become unacceptable, as determined by your oncologist. Some patients take Lenvima for months, while others may take it for years. Your doctor will monitor your response through regular imaging and blood work.
3. Can Lenvima cure thyroid cancer?
Lenvima is not considered a cure for thyroid cancer. It is designed to slow or stop cancer growth and may shrink tumors in some patients. The goal of treatment is to control disease progression and extend the time before the cancer worsens.
4. Why is the thyroid cancer dose of Lenvima higher than other indications?
The 24 mg daily dose for thyroid cancer was established through clinical trials as the most effective dose for this specific cancer type. Other Lenvima indications use lower doses (8–20 mg) based on their own clinical trial data. Your oncologist may reduce the dose if needed based on how you tolerate the medication.
5. Will Lenvima affect my thyroid hormone replacement medication?
Lenvima can affect thyroid function, which is especially relevant for patients who have had a thyroidectomy and are already taking thyroid hormone replacement. Your TSH levels will be monitored monthly, and your endocrinologist may need to adjust your thyroid medication dose during Lenvima treatment.
6. Can I take Lenvima if I have kidney or liver problems?
Patients with mild to moderate kidney or liver impairment generally do not need dose adjustments. However, patients with severe kidney impairment (CrCl less than 30 mL/min) or severe liver impairment (Child-Pugh class C) may need a reduced starting dose of 14 mg daily. Your oncologist will determine the appropriate dose based on your kidney and liver function.
7. Is there a generic version of Lenvima available?
No. Currently, there is no generic version of lenvatinib available in the United States. Lenvima is available only as a brand-name product from Eisai Inc. QuickRx can help you access copay assistance programs to help manage the cost.
8. What if I need surgery while taking Lenvima?
Lenvima should be withheld for at least 1 week before any elective surgery and should not be resumed until at least 2 weeks after major surgery or until adequate wound healing has occurred. The same applies to invasive dental procedures. Always inform your surgeon that you are taking Lenvima.
9. Can I get pregnant while taking Lenvima?
Lenvima may cause harm to an unborn baby. Patients who could become pregnant should use effective contraception during Lenvima treatment and for at least 30 days after the last dose. Pregnancy status should be confirmed before starting treatment. Discuss family planning with your oncologist before starting Lenvima.
10. How can I afford Lenvima for my thyroid cancer treatment?
QuickRx Specialty Pharmacy provides free Lenvima copay assistance for thyroid cancer patients. We help with manufacturer copay cards, patient assistance programs, and foundation grants. Our team handles all paperwork — call (917) 830-2525 or toll-free (800) 496-6111 to get started.
Author: Paola Larrabure, Pharma Content Manager at QuickRx Specialty Pharmacy
Medically Reviewed by: Julia Kravtsova, PharmD, Head Patient Navigator at QuickRx Specialty Pharmacy
Last Updated: February 2026
References
- Lenvatinib. Lexi-Drugs. Lexicomp. UpToDate, Inc. Accessed February 13, 2026.
- National Cancer Institute. Lenvatinib Mesylate — NCI Drug Information.
- National Cancer Institute. Thyroid Cancer — Patient Version.
- National Cancer Institute. Thyroid Cancer Treatment (PDQ) — Health Professional Version.
- U.S. Food and Drug Administration. FDA Approved Drug Products: Lenvima (lenvatinib).
- American Cancer Society. Thyroid Cancer Overview.
- American Thyroid Association. Thyroid Cancer Information.
- Mayo Clinic. Thyroid Cancer — Symptoms and Causes.
- National Cancer Institute. Targeted Therapy to Treat Cancer.
Comprehensive Medical Disclaimer: This article is published by QuickRx Specialty Pharmacy for educational and informational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified healthcare provider with any questions you may have regarding a medical condition or medication. Never disregard professional medical advice or delay in seeking it because of something you have read in this article. QuickRx Specialty Pharmacy does not recommend or endorse any specific tests, physicians, products, procedures, opinions, or other information that may be mentioned in this article.
HIPAA Notice: QuickRx Specialty Pharmacy is committed to protecting your privacy. All patient information is handled in accordance with HIPAA regulations. View our Privacy Policy.
Lenvima® is a registered trademark of Eisai Inc. QuickRx Specialty Pharmacy is not affiliated with Eisai Inc.